The life sciences, medicine, and diagnostics are currently undergoing a similar development as electronics did 60 years ago. The big trends are miniaturization and functional integration. The key enabling technology for these trends is microfluidics, also called Lab-on-a-Chip or, from its historical roots in analytical chemistry, µTAS (miniaturized total analysis systems [1]). Microfluidics is the science and technology of manipulating fluids in functional components with structures in the range from several micrometers up to millimeters. (after G. Whitesides [2]).
What is microfluidics?
Miniaturization is the key
Nowadays, almost no product development in the life sciences or diagnostics (especially molecular diagnostics) takes place, which does not in one form or the other involve elements with microfluidic functionality. The physical principle or scaling law, which presents the foundation of this development, is Fick’s law of diffusion [3], which can be read as:
t = l2/D,
with t the diffusion time (i.e. the time two molecules need to meet each other by random motion), D the diffusion coefficient and l the distance between these molecules (or typical length scale). If l now is reduced (e.g. by a factor of 10) due to the miniaturization of the structure in which these molecules reside, the diffusion time is reduced by a factor of 100! At the same time, also due to this reduction in scale, many more reaction sites can be generated per unit area, which leads to an additional upscaling in the possible information density [4].
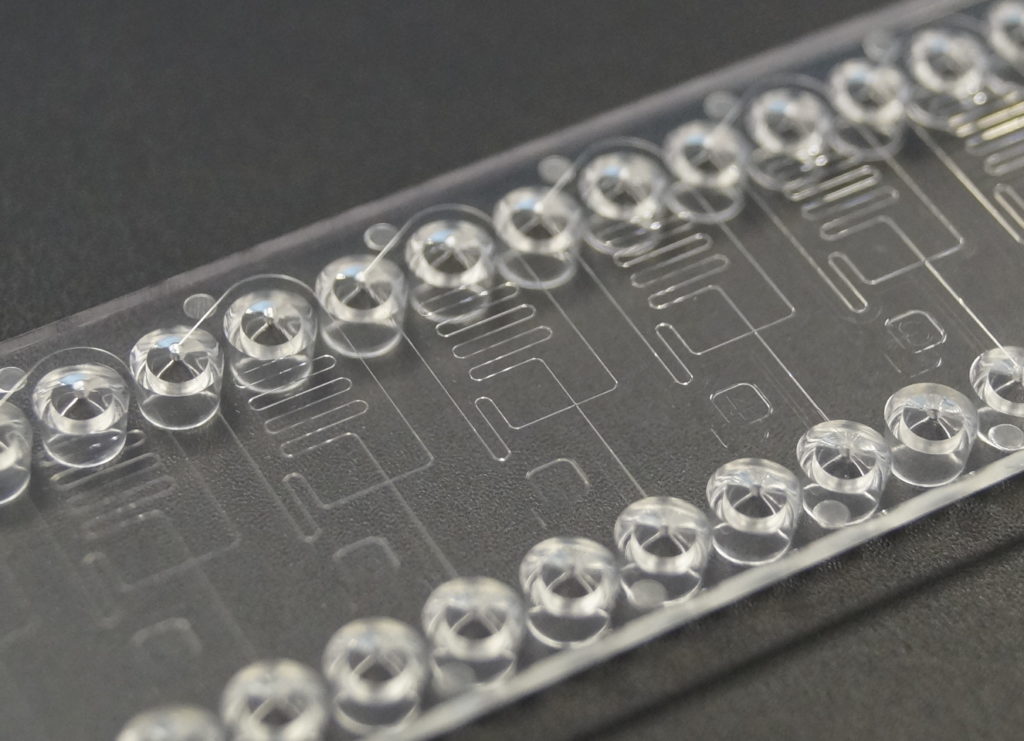
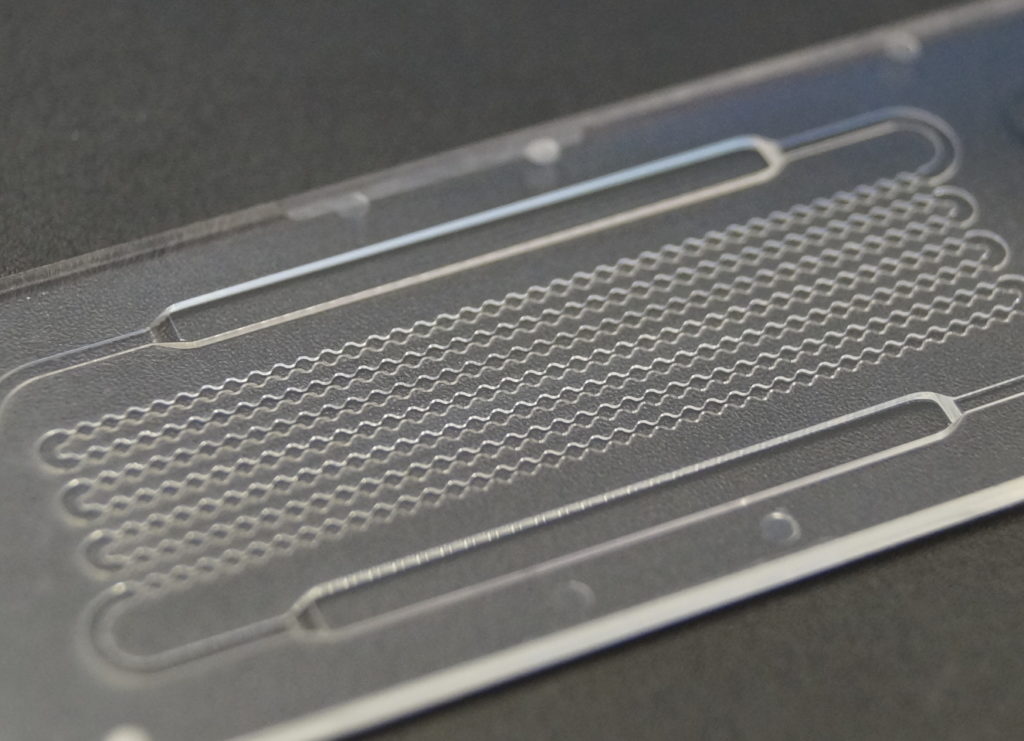
In addition to these performance advantages, miniaturization allows for a reduction in required sample and reagent volumes, which is important, e.g. when there is only very little sample available (e.g. in neonatal diagnostics or the analysis of proteins), or the required reagents for the assay are very expensive. At the same time, also the waste volume will be reduced accordingly.
For commercial developments however, the most important aspect of this miniaturization is the possibility to integrate complex workflows (e.g. from molecular biology) into a single device [5], which allows for a hands-free sample-in result-out operation. The ability to create such integrated microfluidic cartridges and to manufacture them in high volumes has only recently been reached, which explains why the commercialization of microfluidics has been picking up speed for a few years. microfluidic ChipShop offers you the tools and the infrastructure to fully utilize the advantages of the microfluidic technology described above for your product development.
A brief History of Microfluidic Fabrication
Historically, microfluidics and the use as lab-on-a-chip for applications in life sciences or analytical sciences started with technologies being available from semiconductor industries. Consequently, since these technologies were available and allowed for micro-structuring, they were used for the first microfluidic devices. Materials that were applicable to be structured by technologies used in semiconductor industries were glass and silicon. First microfluidic devices, besides ink jet printer heads for non-life science microfluidics, were made from glass and silicon, reaching back to the 1970s with Stephen Terry’s gas chromatograph integrated on a silicon wafer, which was functional but rather expensive. These semiconductor manufacturing technologies have been available at many engineering institutes, thus these disciplines pioneered in microfluidics due to the availability of elaborate, however, usually expensive technologies.
Another manufacturing technology arose by simply taking the micro-structured silicon devices made by the semiconductor technologies and replicating the structures into a soft polymer in a process called casting (often also referred to as “soft lithography”), just by pouring the liquid polymer onto the silicone matrix, hardening it, and removing the soft polymer replicate. This process can be repeated may times, and besides the one-time investment in the silicon master, it is – from an equipment point of view – an extremely low-cost technology. The material used for this process is a special kind of silicone, usually PDMS (Polydimethylsiloxane).
Later on, a merger of micro-technology with injection molding, a conventional fabrication technology used for standard life science plastic lab ware, took place. In order to make this technology available for microfluidics, several challenges had to be overcome. First, micro-structured masters in metals that withstand thousands to several hundred thousand replication cycles had to be generated. Following successful replication, assembling technologies needed to be developed. The current progress in commercialization of microfluidic devices is driven by the adoption of industrial replication technologies in combination with the wide variety of commercially available polymers. Not only enable these recent developments a cost-efficient fabrication but furthermore allow for a great design freedom of microfluidic chips.
Manufacturing Technologies for Polymer-Based Microfluidic Devices
Literature:
[1] Manz A., Graber N., Widmer H.M., Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens. Actuators B 1, 244-248, 1990
[2] Whitesides, G., The origins and the future of microfluidics, Nature, 442, 368-373, 2006
[3] Fick, A., Ueber Diffusion, Ann. Phys. 170(1), 59-86, 1855
[4] Janasek, D., Franzke, J., Manz, A., Scaling and the design of miniaturized chemical-analysis systems, Nature, 442, 374-380, 2006
[5] Becker, H., Gärtner, C., Microfluidics-Enabled Diagnostic Systems: Markets, Challenges, and Examples, in: Microchip Diagnostics: Methods and Protocols, 3-21, Springer, 2017



